Image du Jours -- The Long & The Short
Next Long & Short Image du Jour
About this Long & Short -- #1 of 5.

Particle Tracks (K. Cashion)
Normally, I would not bother with purely autobiographical subjects; however, I was asked why I retired so early. I explained that I had decided my career was completed and it could have only become redundant. I liked having 36 years' experience; I could never have tolerated one year's experience 36 times.
I was asked about the range of measurements I had made and as the conversation developed, I found the subject amusing (as are most subjects when considered in depth).
To the point -- I have five images that may be of interest because they are unique and the common theme is "measurements" or "dimensions."
Some reader may not have a technical background but that shouldn't make any difference because all my readers are unusually smart people. This Image du Jour series is called the "Long & Short (of it)."
My first technical job was in instrumentation in Convair-Ft. Worth's nuclear engineering department. I worked with reactors and radiation sources; designed, built, and calibrated detectors; did the same for the remote manipulation of sources; and I found the general sloppiness of the these measurements fascinating.
Nuclear measurements involve small particles. Beta particles are just high-speed electrons and so have negative charges. They give up most of their energy by collision -- like a billiard ball rolling around a table.
Alpha particles are the nuclei of helium atoms and as such, have two protons (positive charge) and two neutrons (no charge). They are massive (relatively) and slow (sort of) and they give up their energy on contact -- like upon hitting paper, or most anything. Though they do not penetrate much, they are quite dangerous because of their size and charge. They can be swallowed or inhaled. One should not ingest uncontrolled doses of alpha.
Gamma is electromagnetic energy and penetrates skin really well and has no charge, but it gives up its energy by ionizing adjacent atoms -- like the atoms in our bodies -- which our bodies do not appreciate having ionized...or charged.
There are many other particles that can cause us problems but most of these do not exist in nature, or in the sphere we inhabit.
With the small sizes and tremendous number of atomic particles, in today's digital mind-set, we would think such large populations of small particles would somehow contribute to the accurate measurement of their energy, however, this is not the case.
I have exposed a calibrated detector to a known radiation source emitting a known type of radiation, and after 24 hours, recorded a value for the total detected radiation. In the next 24-hour measurement, I might get near-half that first value, or possibly near-half again as much. This is a coarse measurement.
It was difficult to decide what the "real" number was with those two long measurements. Consequently, in this business we had to make a lot of measurements and do a lot of mathematical modeling. It would be better to make 1,440 one-minute measurements (24 hours) than to make one 24-hour measurement.
We would agree that 50% error is a crude measurement.
I left that occupation and started calibrating microwave equipment at Cape Canaveral. The highest frequency I worked was near 30 gHz which is 30,000,000,000 cycles (or events) per second. I calibrated this equipment to within 0.5%.
Now, this is a lot more accurate.
How accurate?
It is within 1 part in 200.
Then I went to NASA. Most of the guys I worked with there had come from Convair's nuclear engineering department. I had to put in a nuclear instrumentation department including a lab with sources and a special scientific photo lab. As this was becoming operable, I started doing my first NASA nuclear work.
In May 1963, the last Mercury mission was flown. This was Mercury-Atlas flight #9 (MA-9).
NASA cancelled what had been planned to be the last Mercury flight, MA-10, because Mercury had completed its mission requirements by the ninth flight. Alan Shepard was to have been the MA-10 astronaut and he was one unhappy fellow when it was cancelled.
MA-9 had been Gordon Cooper's flight and it made 22 orbits at a nominal 100-mile altitude (34 hours). On orbits 17 and 18, background radiation measurements were made for us in the Crew Protection Branch of the Advanced Spacecraft Technology Division.
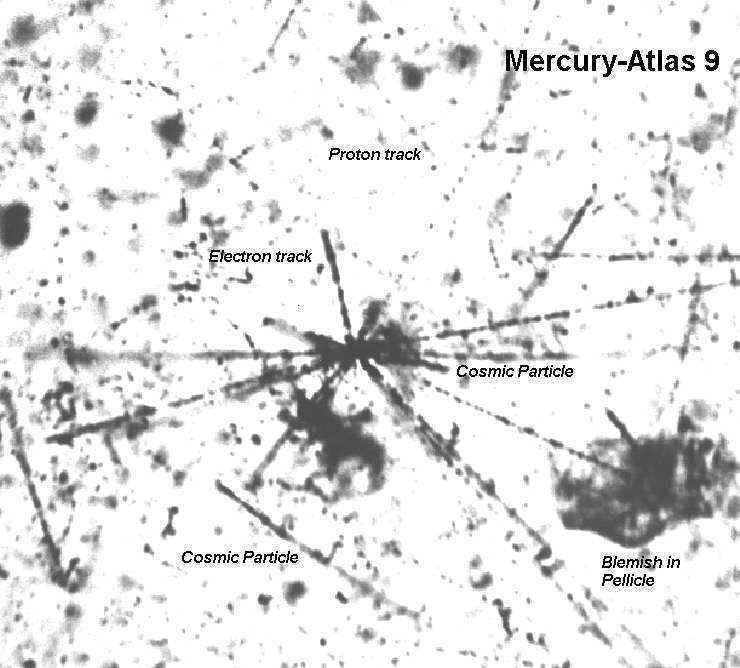
These measurements started with nuclear thick-film emulsions, called "pellicles" on board the spacecraft. The pellicles are like the thin layer of emulsion on the base of a roll of 35mm film, but pellicles might be 1" x 3" and perhaps 0.02" thick (about as thick as 6 sheets of computer paper), and pellicles are pure emulsion; there is no base.
Pellicles are both fun and difficult to process, but I had installed good processing equipment for this. I processed a bunch of test pellicles to make sure it was all set up correctly and I knew what I was doing. After I had processed these early pellicles, I mounted them to standard 1" x 3" glass microscope slides.
These pellicles, while in orbit, had been exposed to ionizing background radiation and this radiation changed the composition of the molecules in the pellicle. After chemical processing like what is done with most light-sensitive film, the tracks left by the particles through the pellicle were obvious -- if the observer knew what to look for.
With a scanning, binocular microscope set to 250 power, I would search (visually) this 1" x 3" slide for the particle tracks. At this magnification, the slide would seem 21' x 62' when viewed just above it by the unaided eye -- and I could only see a tiny part of it at any one time.
Imagine fine, little buffed scratches on a piece of floor 21 x 62 feet, and you are on your hands and knees looking for the faint scratches and then measuring how long they are...but if you don't look for them through a straw, you can't see them. Because floor surfaces are hard and have no depth, this floor you are measuring has many "depths." You not only have to look across the area...but also "down into the floor" -- a floor 5" thick and transparent. That is a good analogy. (When you get very familiar with this floor and you can just zip to a particular place in the floor, you can start learning the details in the other 5" x 21' x 62' floors...maybe a total of 30. They are all different.)
It took several days of scope-viewing to even determine what was there, much less, understand it.
This first Image du Jour is of a photo I made through that microscope and shows the kind of things for which I was looking. This image constitutes nearly one field-of-view at any given time.
The center feature is of a heavy (fast) cosmic particle impacting a silver-iodide crystal. These crystals make up most of the emulsion. The smaller, straight tracks are from protons, and the curly ones are beta particles ricocheting off other crystals' atoms.
The microscope had a very shallow depth-of-focus so there would be many vertical "layers" or "focus planes" the observer would have to use to view the full depth. The image shows just one of these thin "layers." This one is down inside the transparent pellicle someplace. I know this is true because the top or bottom surface cannot be seen in the photo.
I had calibrated this pellicle material and I knew that particles of certain sizes, speeds, and charges (energy) gave up some known amount of energy per known length of pellicle it passed through. This gives a "stopping power" measurement for each particle, and consequently, the energy of the penetrating particle can be determined by measuring the length of its track.
All movement of the image is either x, y, or z (depth). The pellicle was moved by turning three very precise micrometers and reading the changes of position on the barrels of the micrometers.
I have to be careful here to not get into a treatise on nuclear particle tracks -- but the point is, I measured the length of tracks of very small particles. The curly tracks I had to geometrically straighten out to determine their length.
I did quite a few of these pellicles and wrote the technical papers on my findings. These went to the particle physicists assigned to that flight experiment.
So with this first Image du Jour, we have gotten an idea of both the very large and very small measurements one can expect in nuclear instrumentation.
The images will not be seen in the sequence they were acquired.
Ken Cashion
Next Long & Short Image du Jour